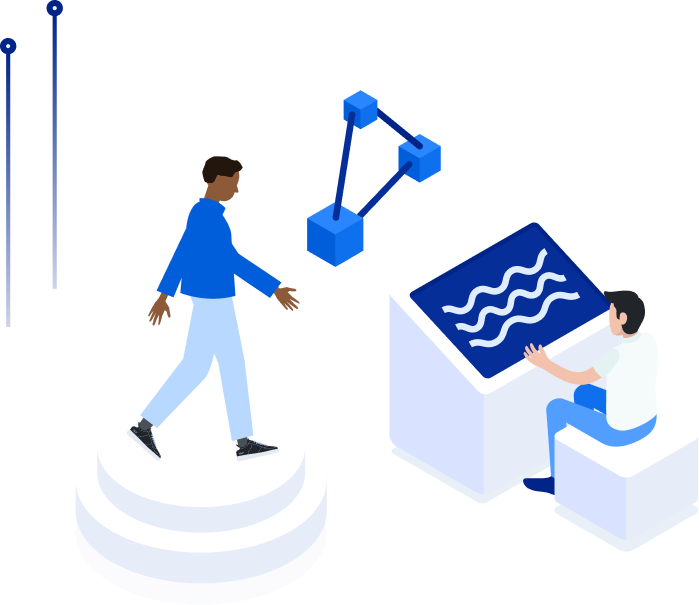
Digital solutions to assess mobility, predict injury and improve life quality.
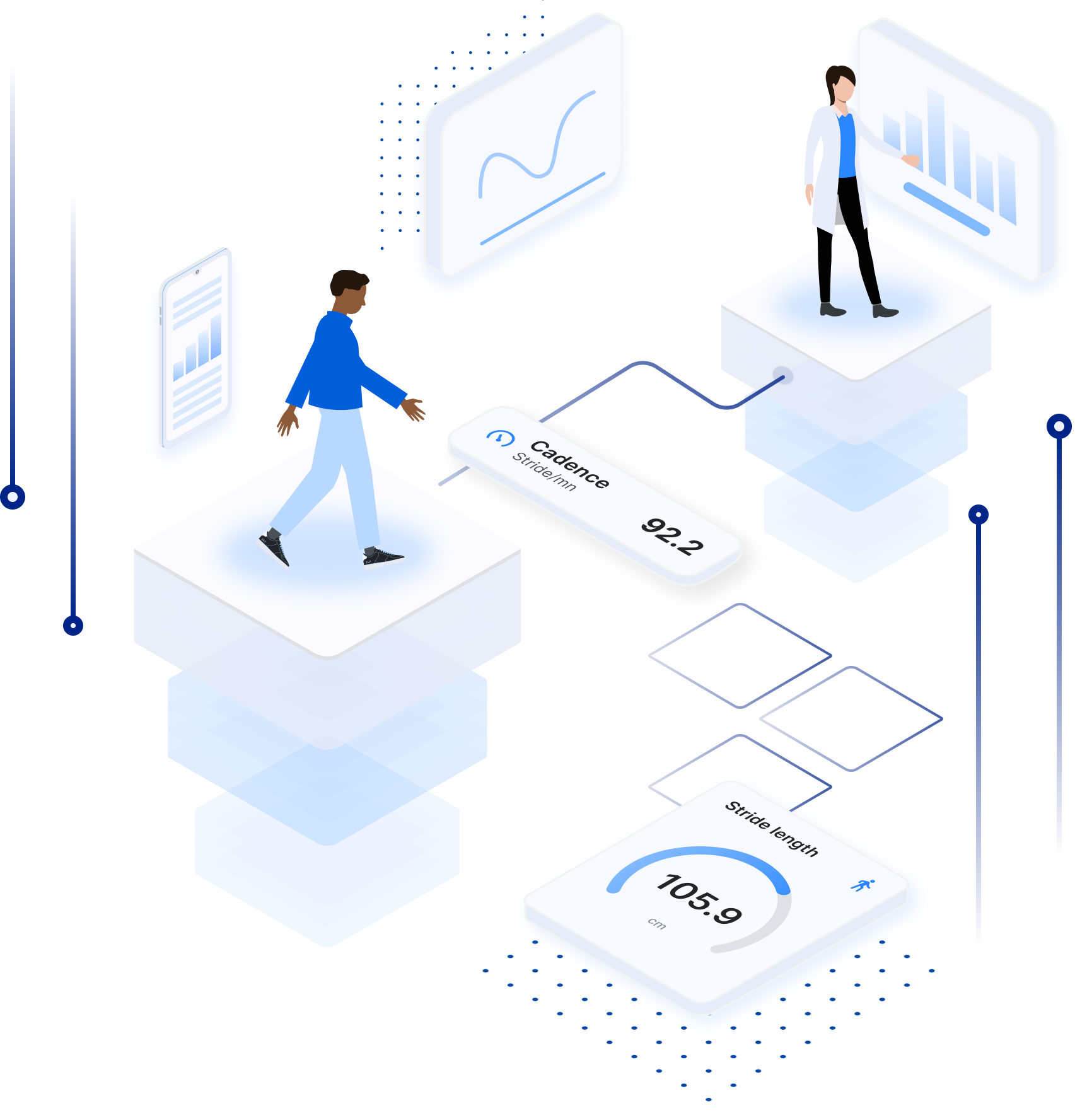

All the power of a professional gait analysis laboratory in medical grade, smart-connected insoles for improved mobility, gait tracking and remote monitoring.
The FeetMe solution provides mobile apps with real-time biofeedback and a web-based dashboard that improve remote monitoring, rehabilitation programs, clinical trials and more.
This powerful gait outcome, like blood pressure, can diagnose an individual’s health status and predict future health status. FeetMe, a medical device company (ISO 9001 and ISO 13485), provides the best solution to improve wellbeing


Real-time gait data collection to assess levels of disability and physiotherapy intervention assistance.

Real-time weight bearing and fatigue alerts, remote consultations and a dynamic digital rehabilitation program.
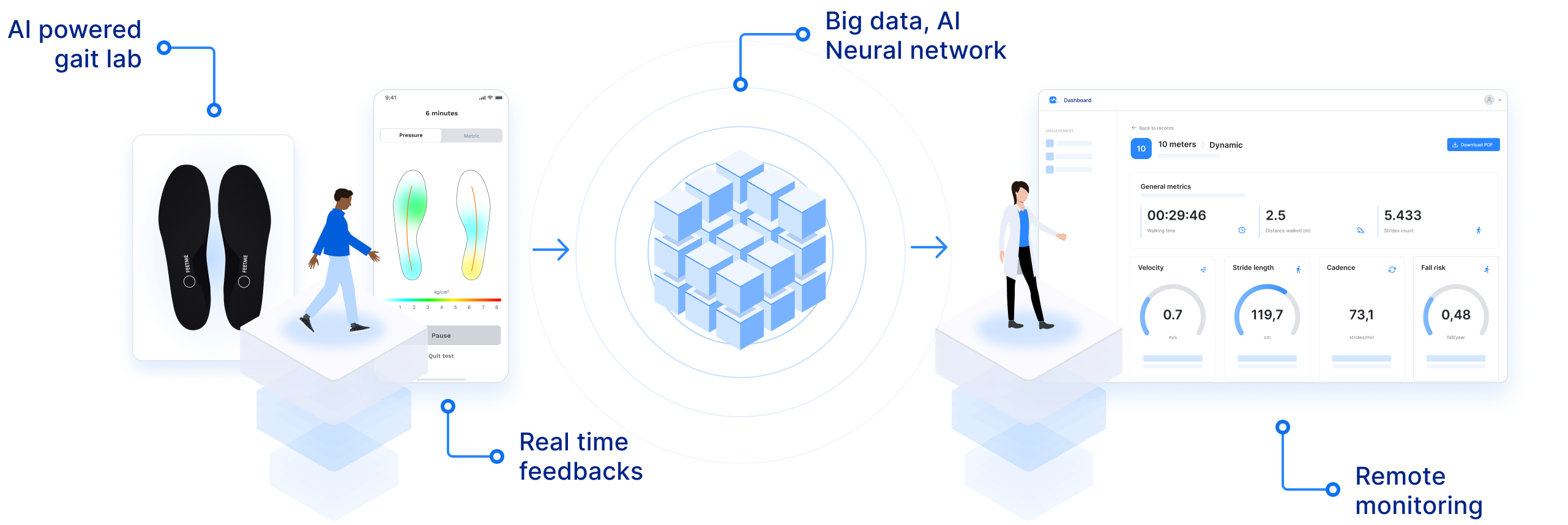
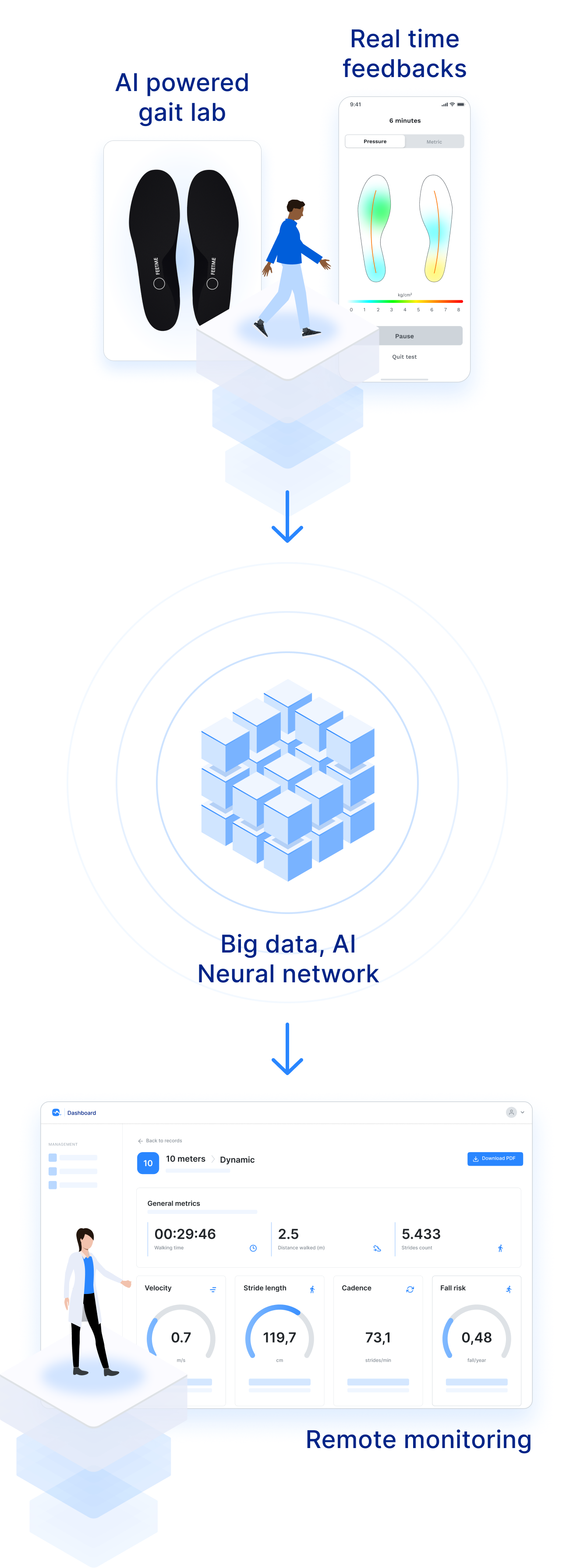
















"FeetMe® Monitor Insoles' pressure and IMU sensors and built-in algorithms maximize patient comfort and speed gait assessment during busy daily clinical practices, ultimately favoring its use in clinics and during long-term home follow-up."

"FeetMe® Evaluation is an accurate and reliable system for measuring gait velocity, stride length, cadence, and stance duration in chronic hemiparesis."

"FeetMe® Monitor Insoles do not require a connexion box connected to a computer to transmit data and have real-time feedback of walking parameters in the phone app."

"To our knowledge, FeetMe® Monitor Insole is the first validated medical device that would allow wearable gait monitoring of Multiple Sclerosis (MS) patients, potentially assessing disease activity, progression and treatment response all at once."
